How it relates to chemistry
Chemistry's Role
Chemistry is used to through out water treatment process to separate harmful chemical found in water. Chemicals such as aluminum sulfate are added to water to reacts with the natural alkalinity in solution to form an insoluble precipitate. Aluminum sulfate collides with the organic matter found in water, "clumps" them together and therefore increasing them in size. This process makes the removal of unwanted substance easier.
Example of chemical reaction
To remove the hardness of water caused by dissolved calcium sulfate, chemical methods are used. The least expensive water softening chemical is washing soda, which is the common name for hydrated sodium carbonate. the addition of sodium carbonate to hard water precipitates the insoluble calcium carbonate. the following equation represent the reaction:
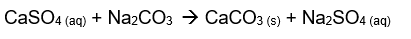
Net ionic equation:
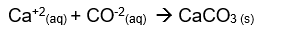
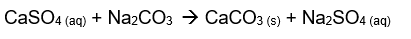
Net ionic equation:
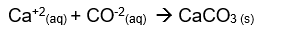
pH Correction
Lime is added to the filtered water to adjust the pH and stabilise the naturally soft water in order to minimise corrosion in the distribution system, and within customers’ plumbing.
Molar Concentration
1.5g of aluminium sulphate per 1 litre of solution
m= 1.5g
converted into moles
n= 0.028mol
V=1L
M=?
c=n/V
=0.028 mol / 1L
=0.0.28M
Therefore there is a concentration of 0.028 mole/L
m= 1.5g
converted into moles
n= 0.028mol
V=1L
M=?
c=n/V
=0.028 mol / 1L
=0.0.28M
Therefore there is a concentration of 0.028 mole/L
